The Kinetic Molecular Theory Helps Explain the Differences Between
The kinetic theory of matter helps us to explain why matter exists in different phases ie. It gives the relationship between pressure temperature and.
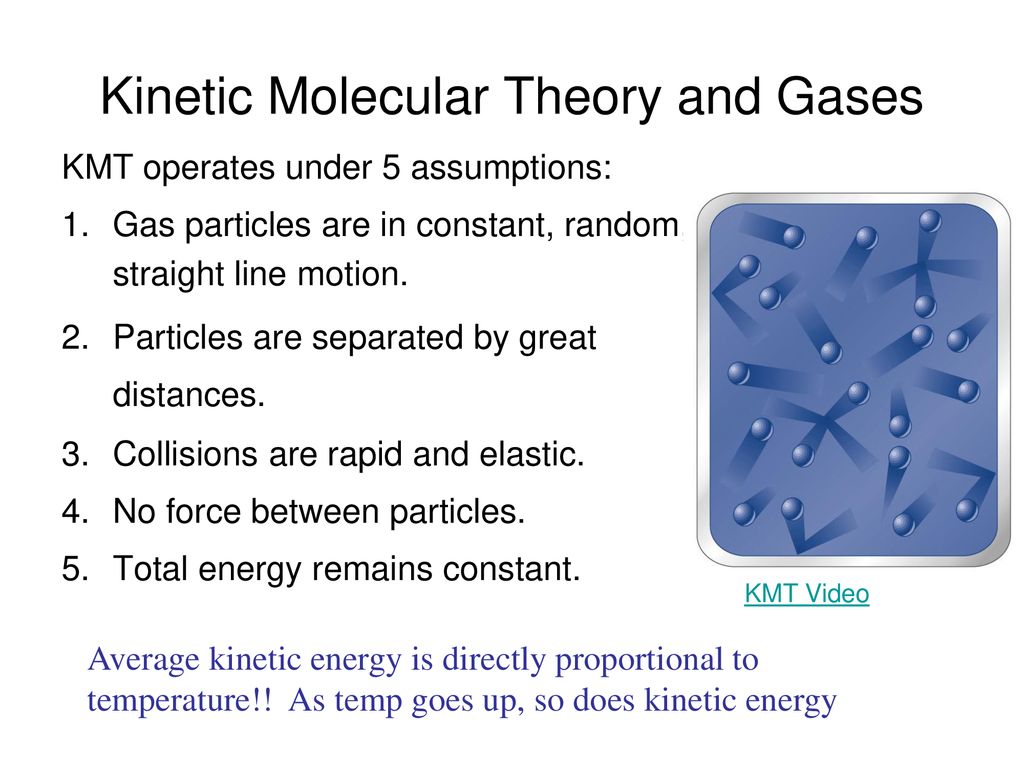
Kinetic Molecular Theory And Gases Ppt Download
The kinetic theory of matter also helps us to understand other properties of.

. The kinetic theory of matter also gives us a description of the microscopic properties of atoms. 32 The kinetic molecular theory ESAAL The kinetic theory of matter helps us to explain why matter exists in different phases ie. The kinetic theory helps to explain the differences between the three common states of matter.
The kinetic molecular theory states that gaseous particles move randomly and constantly. Give two differences between a gas and a liquid and explain these differences with the kinetic molecular theory of gas. Broadly the kinetic theory of matter says that all matter is composed of particles.
At the molecular level the interpretation of pressure and temperature can be explained. It cannot be proved. 10c2 I can use KMT to describe the properties and molecular motion of solids liquids and gases.
Kinetic molecular theory is a theory used to explain physical properties of a gas. Explain the reason for the difference. The Kinetic Molecular Theory helps explain relationships between.
But a law is. 10c1 I can explain the purpose of kinetic molecular theory in my own words. Solid liquid and gas and how matter can change from one phase to the next.
The kinetic theory of matter also helps us to understand other properties of matter. The kinetic theory of matter also helps us to understand other properties of matter. Explain what the difference is between a scientific law and a scientific theory.
The Kinetic Molecular Theory helps explain relationships between. Motion energy temperature phases. Up to 24 cash back Kinetic Molecular Theory By completing this learning activity you should be able to meet the following objectives.
The main purpose of this theory is to explain the existence of matter in various phases and they change from one state to another. The kinetic theory of matter helps us to explain why matter exists in different phases ie. The key difference between particle model of matter and kinetic molecular theory is that the particle model of matter describes the properties of solid liquid and gas phases of matter whereas the kinetic molecular theory describes the properties of gases.
The Kinetic Molecular theory is used to describe the behavior of gas. Energy conversion between kinetic and potential energy. The average distance between the molecules.
The Kinetic Molecular Theory helps explain relationships between. The kinetic theory of matter helps to explain the differences between _____ A. The Kinetic-Molecular Theory Explains the Behavior of Gases Part II According to Grahams law the molecules of a gas are in rapid motion and the molecules themselves are small.
That of ethylene glycol CH2 OHCH2OH is 6208 yet their boiling points are 1172 C and 174 C respectively. The average kinetic energy of the particles decreases as the particles go from a liquid state to a gas state because gas particles do not Chemistry According to the kinetic-molecular theory gases are highly compressible because the particles of they consist. Lose energy when they collide with each other b.
Matter is made up of particles that are constantly moving. Kinetic theory explains that. Particles of only a gas.
All matter is made of particles called atoms B. The existence of more hydrogen bonds. Gravity temperature nuclear forces.
Solid liquid and gas. A scientific theory predicts why something might happen and when more information goes into the theory it can better predict. It is important to realise that what we will go on to describe is only a theory.
It gives the relationship between pressure temperature and. Although the molecular kinetic theory was born from the study of the gaseous state since there were many studies on it that allowed to write the ideas it also serves to explain the constitution of liquids and solids. Which of the following is not a postulate of the kinetic molecular theory.
Solid liquid and gas and how matter can change from one phase to the next. Which of the following is not a statement regarding the Kinetic Molecular Theory of Matter. Solid liquid and gas and how matter can change from one phase to another.
All particles have energy but the energy varies depending on the temperature the sample of matter is in. The kinetic molecular theory of matter states that. The Kinetic Molecular theory is used to describe the behavior of gas.
Moreover it offers a way to see differences between the different states of matter. The two hydroxyl groups in ethylene glycol provide more locations for the formation of hydrogen bonds. And describe how particles are behaving during a phase change.
Difference between the states of matter. The kinetic molecular theory. Moreover it also stats that there is no loss of kinetic energy when particles of.
All of the above. This in turn determines whether the substance exists in the solid liquid or gaseous state. Are very apart from each other c.
Conversion of mass to energy to mass. Use the kinetic molecular theory matter particles to explain the differences between solid vs liquid vs gas phase. The molecular mass of butanol C4H9OH is 7414.
Give two differences between a gas and a liquid and explain these differences with the kinetic molecular theory of gas.
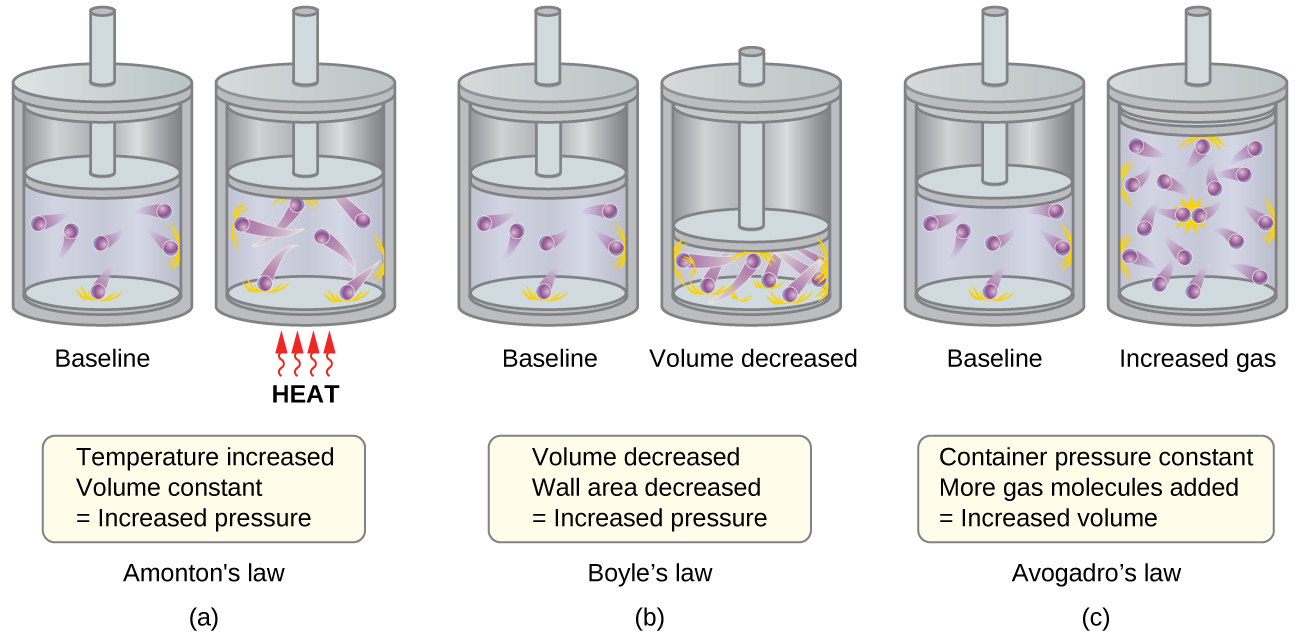
9 5 The Kinetic Molecular Theory Chemistry
No comments for "The Kinetic Molecular Theory Helps Explain the Differences Between"
Post a Comment